Near-Infrared AOTF Spectroscopy for Controlled Release Tablets
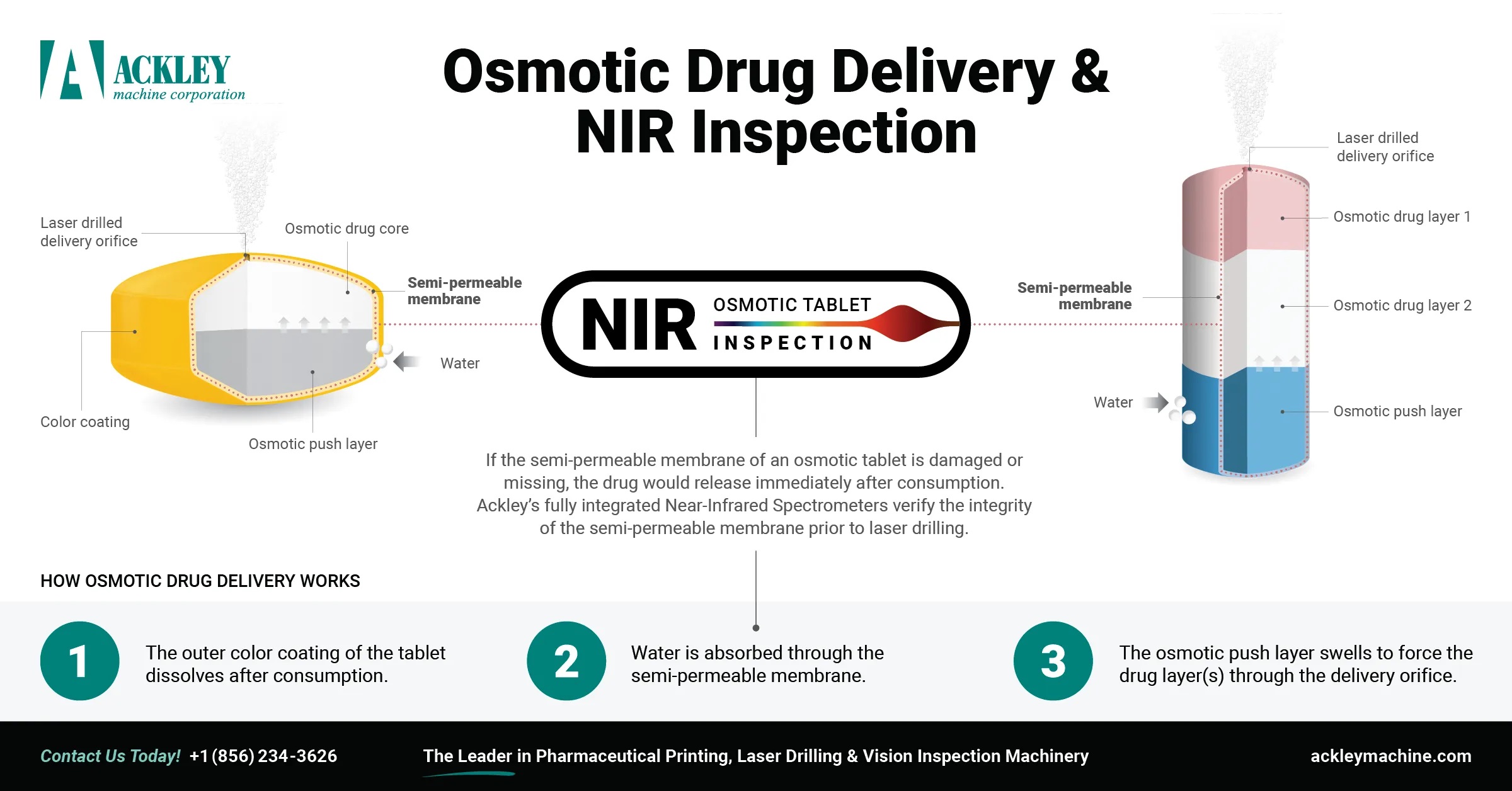
Osmotic drug delivery has been simplified with the addition of NIR AOTF spectroscopy to Ackley’s line of pharmaceutical tablet laser drilling machines.
The osmotic-controlled release oral delivery system, or OROS, is an advanced timed release mechanism usually in the form of a bi-layer tablet with a semi-permeable outer membrane which retains its integrity until it reaches the intestines. If this coating is damaged, the drug might release immediately after ingestion, which could be very harmful, or even fatal, to the consumer.
NIR Inspection provides a quick and accurate way to verify that a tablet’s enteric coating is acceptable and therefore safe for consumption. Near-infrared spectroscopy provides a unique advantage for pharmaceutical manufacturers due to its ease of use and fast, accurate analysis of solid dosage forms. This analytical technique uses the near-infrared region of the electromagnetic spectrum to provide a material signature that can be plotted to show peaks at specific wavelengths of light. This analysis provides information on a material’s composition and can provide important information about the safety and quality of coatings on OROS tablets.
Ackley’s CO2 precision laser drilling machinery is now equipped with optional near-infrared spectroscopy in addition to vision inspection. Our Class 1 Laser tablet drilling machines drill sub-millimeter sized apertures into the enteric coating of single and bi-layer tablets to achieve the desired dissolution rate and drug release profile. This fully-integrated NIR inspection system provides and increased level of safety for consumers and additional peace of mind for pharmaceutical manufacturers by giving them twice the inspection power, ensuring that only the best products make it to market.
Whatever your laser drilling needs, our engineering team is ready to design a solution that will give your controlled release product a perfectly aligned, precisely drilled aperture, each and every time.